-
- OUR PRODUCT
 R&D Pipeline
R&D Pipeline Product List (Download)
Product List (Download)
 R&D Pipeline
R&D Pipeline
| ODF Products | Development Status | |||||
|---|---|---|---|---|---|---|
| Therapeutic Class | Active Ingredient(s) | Indication | Formulation study | BE study | Product Approval | Launch |
| Urology | Sildenafil Citrate | Erectile dysfunction |
 |
 |
 |
 |
| Launched with the brand name of Viagra L in 2013 | ||||||
| Urology | Tadalafil | Erectile dysfunction |
 |
 |
 |
 |
| Launched with the brand name of Vulteum in 2014 | ||||||
| Neurology | Donepezil HCl | Alzheimer's disease |
 |
 |
 |
 |
| Launched with the brand name of Artpezil in 2016 | ||||||
| Nutrition | Vitamin complex | Vitamin supplements |
 |
 |
 |
|
| Approved by MFDS in 2018. | ||||||
| Urology | Solifenacin Succinate | Overactive bladder |
 |
 |
 |
|
| Will launch with the brand name of OBCARE ODF in 2018 | ||||||
| Neurology | Aripiprazole | Schizophrenia |  |
|||
| Endocrinology | SPO-1405 | Obesity |  |
|||
| Nutrition | SPO-1705 | Tonic |  |
|||
| Nutrition | SPO-1706 | Vitamin supplements |
 |
|||
| OB/GY | SPO-1801 | Menopausal syndrome |
 |
|||
 Product List
Product List Download

-
Brand Name. VULTEUM ®
Active ingredient. Tadalafil 5mg / 10mg / 20mg
Indication. Erectile dysfunction
Packing. 14 films / box (5mg) / 10 films / box (10mg, 20mg)
Shelf life : 3 years
-
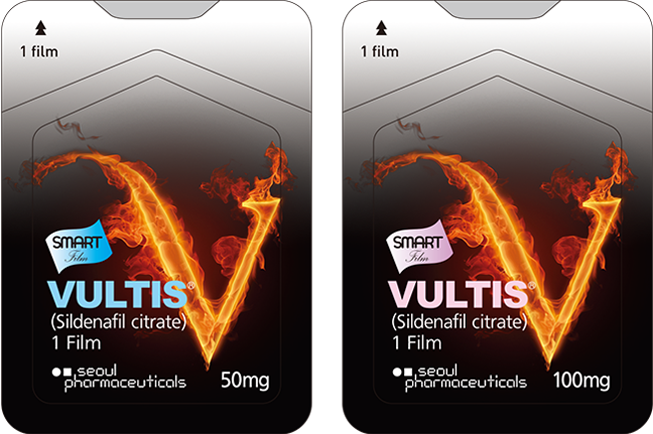
Brand Name. VULTIS ®
Active ingredient. Sildenafil Citrate 25mg / 50mg / 100mg
Indication. Erectile dysfunction
Packing. 4 films / box
Shelf life : 3 years

-
Brand Name. ARTPEZIL ®
Active ingredient. Donepezil HCl 5mg / 10mg
Indication. Alzheimer’ disease
Packing. 30 films / box
Shelf life : 3 years
-
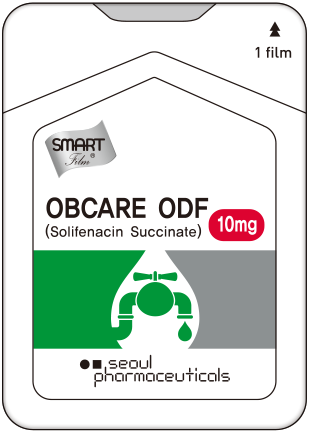
Brand Name. OBCARE
Active ingredient. Solifenacin Succinate 10mg
Indication. Treatement of overactive bladder with symptoms of urge urinary incontinence, urgency, and urinary frequency

-
Brand Name. (Upcoming)
Active ingredients. Niacin, Thiamine nitrate, Riboflavin
Indication. Vitamin B supplement


